Flow batteries - A disruptive force in energy storage with the industrialization of a new chemistry
 Can renewable energy replace fossil fuel or nuclear power plants?
Can renewable energy replace fossil fuel or nuclear power plants?
Even if it doesn't happen overnight, the future of energy will not be limited to just our good old power plants. And it's not because we've suddenly decided to tackle global warming. Many studies illustrate what's happening all over the world: renewable energy is successfully competing with fossil fuels and nuclear energy, irrespective of public incentives.
This is why stationary energy storage has drawn so much interest in the last few years. If wind can be stored at night or solar energy during the day, to be redistributed when it is needed, it will allow an incremental rise in production, removing the last roadblock to a fully green energy mix.
This also explains why flow batteries have recently attracted hundreds of millions of dollars in investment worldwide. Storing renewable energy, or related grid management services, require a power range of dozens of kW to several MW, several hours of charge or discharge, and industrial features such as reliability, easy maintenance and durability. Why are flow batteries so good at doing this?
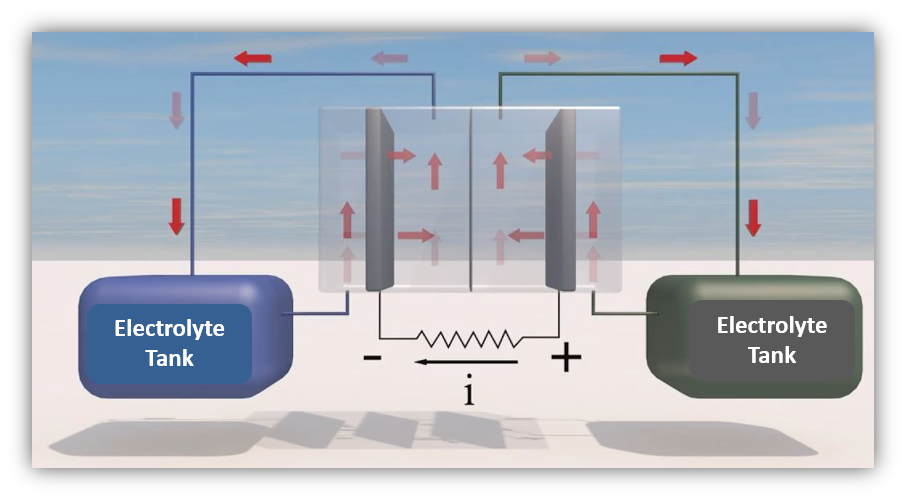
A conventional battery is a box containing electrodes, and liquids called electrolytes. In the process of charging and discharging the battery, the electrode typically dissolves in the electrolyte and is regenerated in the second part of the cycle. The amount of power a battery can store is proportional to the amount of electrolyte in the battery.
Flow batteries are different in two respects. First, the electrolytes are located in tanks outside the battery, and pumped into it for charging and discharging. Flow batteries store outside the box. And second (for genuine flow batteries as opposed to hybrid systems), there is no electrochemical reaction between the electrodes and the electrolytes. Only the molecules in the electrolytes are oxidized and reduced during the cycles; the electrodes serve as electron-carriers.
Two major consequences result. One is that the amount of energy stored in the battery is proportional to the quantity of electrolytes in the tanks, not the size of the battery. For example, in order to store the energy produced by a solar farm, doubling the capacity with conventional batteries (such as lead-acid or lithium-ion) will require double the batteries. Flow batteries only require pouring more electrolytes in the tanks. The other consequence is an increase in battery life. The dissolving and regeneration of the electrodes in ordinary batteries usually leads to a degradation of the performance, and ultimately the demise of the device. The liquid phase reaction in flow batteries is more reversible.
Flow batteries have been developed for several decades, but are still produced in small numbers. What is limiting their expansion?
The energy storage market can take some of the blame. Until recently, it was possible to manage renewable energy without storage, or with short-duration batteries. Flow batteries are unsuitable for other applications such as electronics or electric vehicles because of their low energy density.
The history and evolution of flow batteries has also affected production. The first molecules used in the electrolytes were metals and halogens dissolved in very acidic electrolytes. Today, the dominant chemistry is vanadium (zinc-bromide is another). The resulting electrolytes are extremely corrosive, which can impact reliability, maintenance, safety, and the environment. Vanadium systems use the same electrolyte on both sides, while zinc-bromine systems are more energy-dense hybrids. Both of these smart chemistries, however, are handicapped by the difficulties of chemical engineering, i.e. their implementation in industrial systems.
Various chemistries (with solvents other than water) have been studied in order to prevent these problems and increase performance, but they have not yet reached high Technology Readiness Levels (TRLs).
The technical limitations of the flow batteries have, so far, not completely convinced end-users.
The recent advent of organic flow batteries, on the other hand, can be the game-changer the market needs. Based on organic molecules in aqueous alkaline solutions, this second generation of flow batteries holds the keys to several groundbreaking advances:
- The electrolytes are non-corrosive, eliminating leakages and the need for part replacement;
- The molecules are biodegradable, and potentially reusable or recyclable; solving toxicity, environmental, or life-cycle issues;
- The electrolytes can use off-the-shelf molecules synthesized for other purposes, and at low, non-volatile costs.
Moreover, this new chemistry can apply to entire families of molecules, not just a single element like vanadium or bromide. Future breakthroughs are possible with new molecules, new production processes, or new formulations of the electrolytes.
Industrial scale-up could be surprisingly fast. The process is a combination of well-known technologies, so building a gigafactory is not necessary, and risks are limited. Compared to a lithium-ion battery, flow battery cells are constantly cooled by the flow of water-based electrolytes and cannot burn, and the Battery Management System (BMS) is quite simple.
As the need for long-duration systems increases, either for grid services in advanced economies or off-grid applications such as rural electrification, these new flow batteries may be the key to helping us keep the promises of green energy.
 François Huber is chief executive officer and co-founder of Kemwatt Energy. With over 20 years of experience in renewable energy, electrical equipment, and industrial systems, he established Kemwatt in 2014. Based in Rennes (France), Kemwatt designs and develops organic Redox Flow Batteries using only organic electrolytes. With over €2 million from initial seed funds along with numerous awards, Kemwatt aims to bring their technology worldwide to support all smart and micro grids.
François Huber is chief executive officer and co-founder of Kemwatt Energy. With over 20 years of experience in renewable energy, electrical equipment, and industrial systems, he established Kemwatt in 2014. Based in Rennes (France), Kemwatt designs and develops organic Redox Flow Batteries using only organic electrolytes. With over €2 million from initial seed funds along with numerous awards, Kemwatt aims to bring their technology worldwide to support all smart and micro grids.
Kemwatt Energy | www.kemwatt.com
Author: François Huber
Volume: 2017 January/February









