Corrosion in Solar Support Structures
A topic of increasing interest in the fast-growing solar industry is corrosion abatement in solar support structures.

Corrosion is the deterioration of a material that results from a reaction with its environment. Experts estimate the economic impact of corrosion on an economy to be 3-6 percent of a country’s gross national product (GNP). According to the article "Economic Effects of metallic Corrosion in the United States", published in 1978 by the Battelle Columbus Institute and the NIST, contains calculation to determine the economic effect (equations), which was then used on data from 1995 to determine that year’s cost of corrosion: nearly $300 billion, or ~4.2 percent of GNP.
Since this early economic assessment, new coating technologies have been developed and applied to combat corrosion. These include metallic coatings such as zinc and zinc alloys, polymer-based coatings and paints, epoxies, sol-gel coatings, and hybrid coatings with high solids content applied by liquid or dry powder spray techniques.
Additionally, construction, assembly and building practices have evolved to combat corrosion, as has component design. This is particularly true for the automotive and construction industries. Designs are increasingly engineered for easy water runoff and drying, and there has been a significant reduction in metallic seams, laps, and joints where water, dust, and other contaminates can create corrosion cells.
Figure 1 below depicts a solar pile, to which solar modules would be attached, where it is driven into the ground. Three primary zones of corrosion are shown – atmospheric, ground level, and below ground – each with differing rates of corrosion.
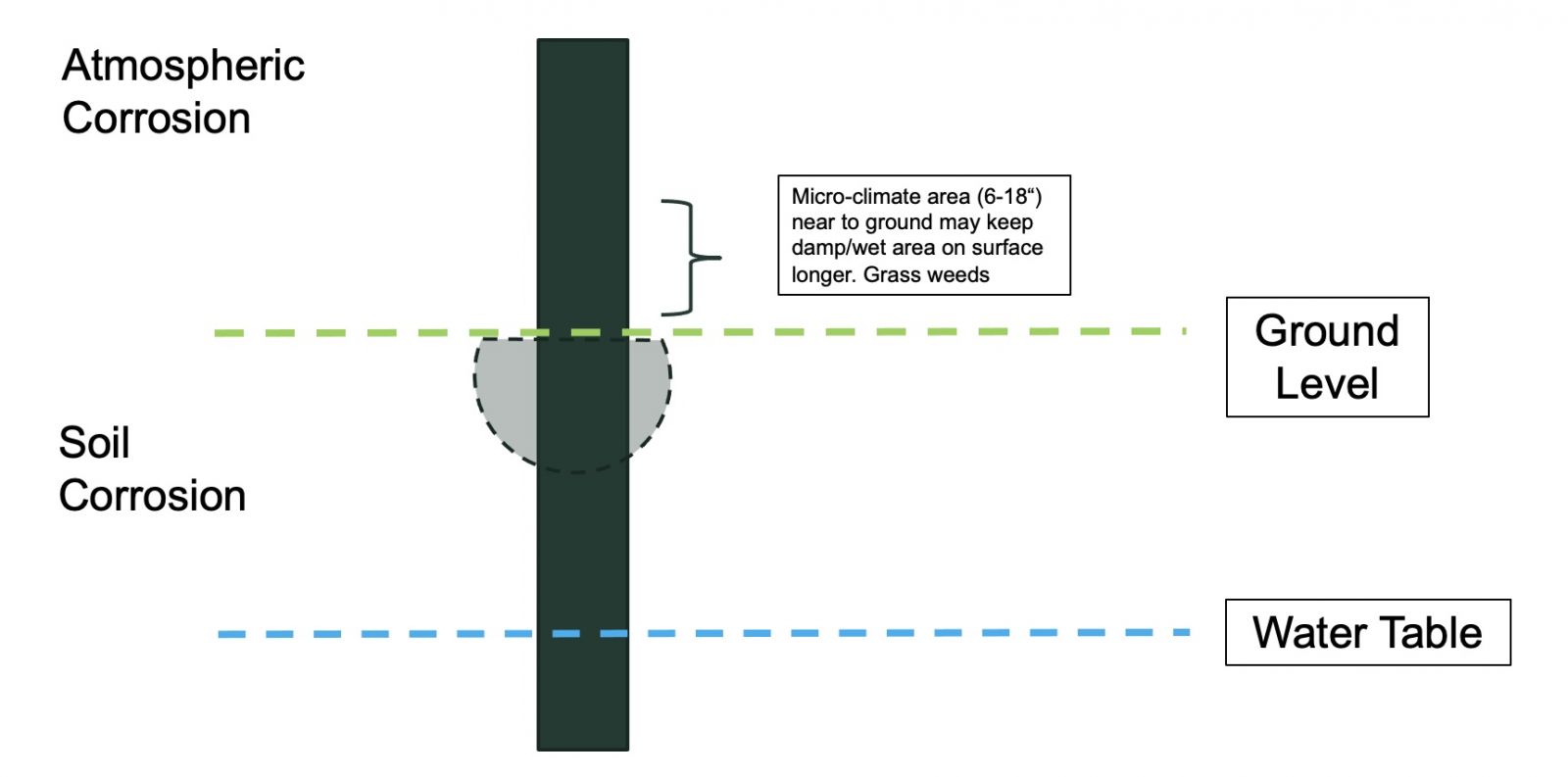
Figure 1. Concept drawing showing various zones of corrosion.
Atmospheric
In atmospheric corrosion, one should consider the location-based wet-dry cycles due to normal weather patterns. Locations near the ocean (< 2 kilometers) may be subject to chlorine ions from salt spray. In locations near industrial areas and highways, pollutants like sulfur increase atmospheric corrosion. Furthermore, higher ambient temperatures increase corrosion rates.
Air-Soil Interface Zone
The air-soil interface zone is the area just above and just below the soil line. The area up to 18” above the soil line can be subject to extended time of wetness (TOW) during certain weather patterns, where warm air condenses on the cold ground surface. In addition, a ‘splash zone’ caused by rain can drive soil particles (or other contaminates) that adhere to the surface and initiate localized corrosive cells. The growth of plants, weeds, and grasses can create a micro-climate near the ground surface that extends the TOW and promotes corrosion.
Below the Surface
The area 10” - 12” below the surface is the most stressful for a solar structure. Subterranean corrosion is also complicated as it must consider soil morphology and atmospheric conditions, which jointly contribute to TOW. Additionally, wind and snow loads create a stress area approximately 6 inches below the soil surface, resulting in corrosion potential. Other factors include poor drainage of soils, and soil disturbances that introduce oxygen, backfill, pH, redox potential, and sulfide content.
Soil resistivity is a significant factor for determining corrosion in soils. High conductivity/low resistivity soils (0-500 ohm-cm) are much more corrosive than soils with high resistivity (> 10,000 ohm-cm).
Electro chemical corrosion reactions are common in steel. It occurs when four requirements are met:
- An anode (where oxidation of the metal occurs)
- A cathode (where reduction of a different species occurs)
- An electrolytic path for ionic conduction between the two reaction sites.
- An electrical path for electron conduction between the reaction sites.
Figure 2 shows a simplified view of a corrosion cell for steel. The anodic and cathodic reactions are shown, and the water droplet is the electrolyte that allows ionic transport of iron (Fe) to be reacted with available oxygen. The steel substrate acts as the electrical path for conduction of electrons.
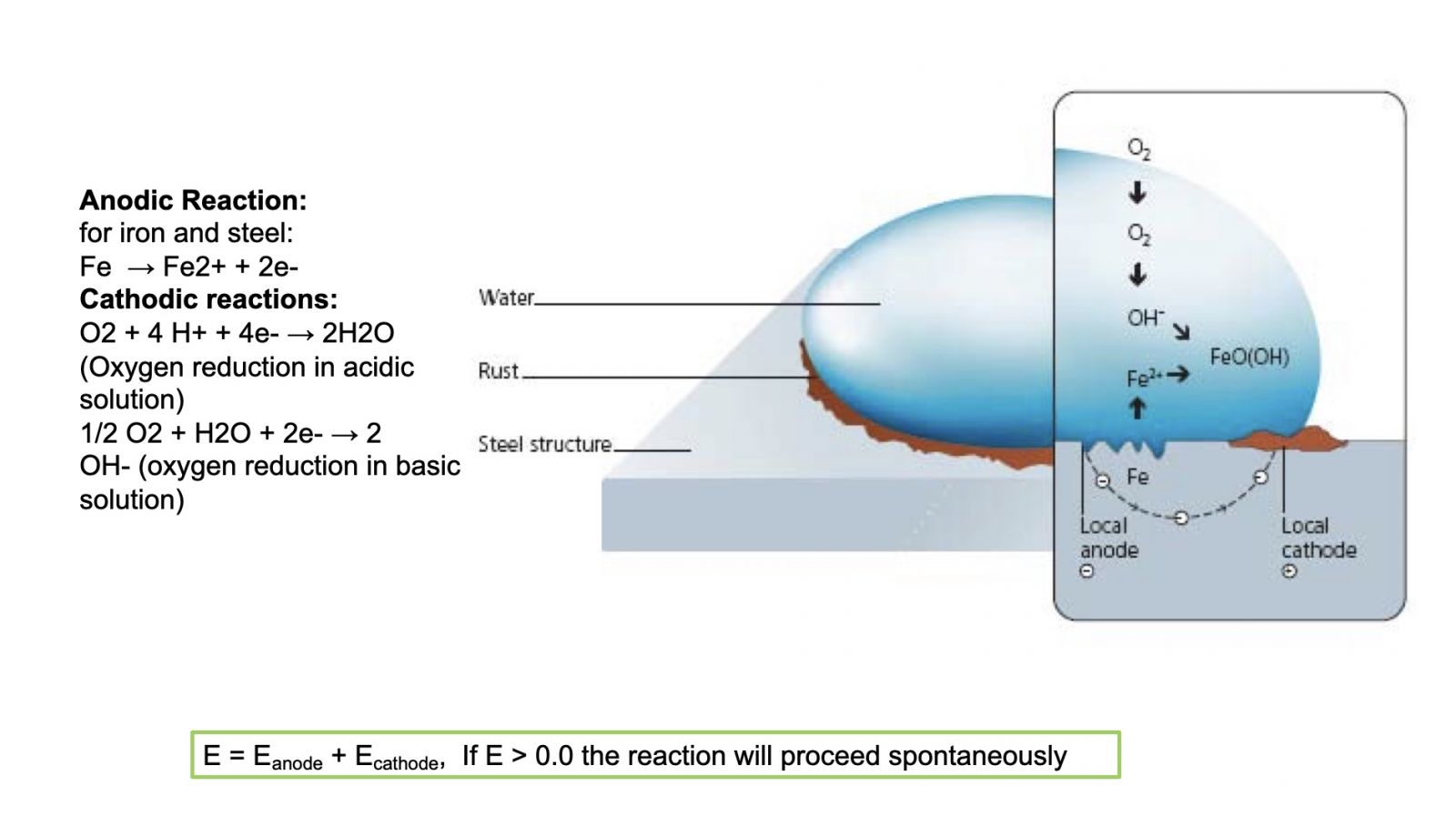
Figure 2. A simplified view of a corrosion cell on steel.
Base chemical compositions can also affect corrosion, including microstructure, inclusions, heat treatment, welding, and weldments.
Environmental factors affecting corrosion include environmentally induced stress or stress induced corrosion, Hydrogen induced cracking, Microbial attach, galvanic or stray current that can induce corrosion. Mechanical scratching and abrasion can also create sites for corrosion initiation.
Atmospheric Corrosion can be estimated by using the widely accepted ISO Standards 9223 and 9224. There is no widely accepted method for estimation of soil corrosion, and it is recommended that a certified corrosion engineer be contacted to estimate the corrosion at your chosen site.
Mark Schlichting is a metallurgical engineer with Nucor Corporation. Mark holds a Bachelor of Science in Metallurgical Engineering from the University of Wisconsin, Madison. He holds 26 patents, mostly in steelmaking, vacuum metallurgy, ironmaking, and casting. Mark has published several technical papers in respected journals including AISE, ISS and Stahl & Eisen.
Nucor | www.nucor.com
Author: Mark Schlichting
Volume: 2022 September/October










.png?r=1181)

