The Path to Hydrogen: Producing clean, storable fuel from biomass
 New methods of hydrogen production are being deployed, which are helping increase the efficiency of biomass facilities, creating high value, clean energy from sources of waste. Seems only natural, considering hydrogen is at the core of every energy source—from the sun that shines upon us, to the gas that powers the majority of our cars.
New methods of hydrogen production are being deployed, which are helping increase the efficiency of biomass facilities, creating high value, clean energy from sources of waste. Seems only natural, considering hydrogen is at the core of every energy source—from the sun that shines upon us, to the gas that powers the majority of our cars.
Thanks to older, dependable technologies that are simply being used in new, unconventional ways, biomass can now easily be converted to hydrogen. This means that in large-scale anaerobic digesters and other biogas projects (including landfill gas), it’s possible to maximize the utility of natural gas products by converting it to high-purity hydrogen fuel. In modern gasification plants, operators will effectively be able to take biomass and purify it into a rich, carbon-neutral fuel source.
Hydrogen from anaerobically digested biomass
Early-stage development of several projects in the Midwestern United States shows a growing interest in generating more advanced fuels from renewable resources. Using chaff (corn and crop byproduct) from fields to produce biogas in an anaerobic digester, farmers now have the primary fuel stock for creating hydrogen, using steam reformation (SMR) technology.
The process looks like this: through an anaerobic digester, methane is created from biologically treated biomass material like chaff, wood pulp, trimmings, dung, biomass garbage, etc. Then, using steam methane reforming equipment, the methane is purified to become high-purity hydrogen fuel, which is a truly clean, high-energy fuel.
The benefit of producing hydrogen is contained in the differences in heating value between hydrocarbon gases, found in natural gas, and the much purer hydrogen fuel product. Whereas natural gas has a relatively low heating value as far as fuels are concerned—methane contains about 23,000 BTU/lb and is the primary component of most natural gas feedstocks—hydrogen fuel contains nearly three times that value within the same mass.
Whether planning to produce electricity, heating, or chemicals from a biomass facility, hydrogen is inherently more productive than methane and other hydrocarbon gases.
The hydrogen fuel product is also cleaner than natural gas produced from most biomass and anaerobic digestion facilities. Using advanced gas reforming technology, an operator of a natural gas plant can easily remove the carbon stream from their production line. Aside from the benefit of creating an end product, which is more uniform and of higher quality (just think, for comparison’s sake, of high octane fuel versus low octane fuel), there’s also legislative benefits in terms of carbon taxes—enabling biomass plants to generate additional revenue from carbon taxes.
Hydrogen from syngas
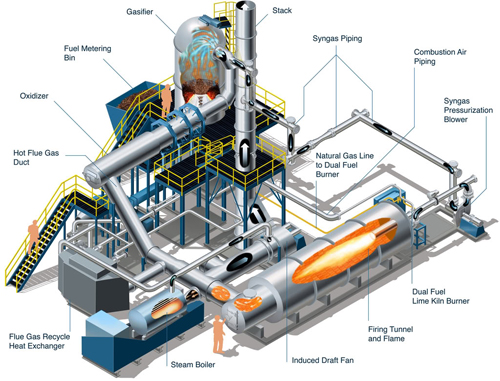
Another potential application of hydrogen technology in the biomass industry, which is proving interesting to stakeholders, is its application to gasified biomass facilities. Biomass gasification turns biomass, such as chaff, wood pulp, or any biomass material, into a gasified product called syngas. Syngas is primarily hydrogen, carbon monoxide, and water. Using phase shifting equipment, which has been standard in the petrochemical industry for over 20 years, biomass gasifiers can maximize the amount of energy created from their biomass feedstock by turning excess carbon monoxide into hydrogen fuel.
Using a simple set of equipment at the end of a gasifier’s line, a gasification plant can turn a toxic pollutant into a highly useful and clean fuel. In this case, however, there is a catch. This equipment does have an intermediary bi-product of CO2. Fortunately, most modern equipment manufacturers can easily equip carbon sequestration onto a gasification facility at small, additional cost.
High growth, great profits
After a digestion facility or a gasification facility is equipped with optimized hydrogen generation equipment, the plant has a great range of potential users. Apart from the traditional demand of hydrogen in the thermal power industry, chemical manufacturing, fertilizer manufacturing, electronics industry, and many other heavy industries, there’s a rising demand for hydrogen in the emerging fuel cell industry.
According to a new report by Frost and Sullivan, major automobile OEMs are planning to release at least a 5,000 fuel cell vehicle (FCV) by 2015 in the US, including about 58,000 FCVs in Japan and Korea. Moreover, many utilities and businesses are now capitalizing on the benefits of stationary fuel cells to provide power, heating, and cooling to their assets. Fuel cell users are increasing rapidly, and will need a steady supply of hydrogen fuel to feed their new demand. Successful manufacturers of hydrogen generation equipment must be adaptive of the market demand, providing flexible solutions that can produce hydrogen gas on small and large scales—and with feedstocks that aren’t only traditional (like natural gas), but also modern (like biogas, syngas, and renewable electricity.)
The main purpose of creating hydrogen in any biomass facility is to maximize the value derived from the biomass resource. By mass, hydrogen creates a very large amount of energy: 120MJ/kg versus 60MJ/kg for methane, the primary component of natural gas/biogas.
Where costs used to be a concern, technology is stepping forward with financially sound solutions. It is now economically feasible to generate hydrogen fuel from existing feedstocks, including biomass, biogas, or syngas. The first facilities have emerged in Europe and Canada, creating hydrogen commercially from renewable resources. It is only a matter of time when demand for the only truly clean, storable fuel will force the hydrogen economy into mass adoption.
Angstrom Advanced Inc. specializes in designing and deploying residential, commercial, and industrial-scale hydrogen electrolysers and hydrogen steam methane reformers used for biofuel processing, renewable energy storage, distributed generation, and more.
Angstrom Advanced Inc.
www.angstromadvancedinc.com
Author: Samuel Sterling
Volume: January/February 2013











