Using Atmospheric Carbon Dioxide: For energy storage systems & products
 Chemists and engineers at Oregon State University (OSU) have discovered a new way to take some of the atmospheric carbon dioxide that’s causing the greenhouse effect and use it to make an advanced, high-value material for use in energy storage products.
Chemists and engineers at Oregon State University (OSU) have discovered a new way to take some of the atmospheric carbon dioxide that’s causing the greenhouse effect and use it to make an advanced, high-value material for use in energy storage products.
This innovation in nanotechnology won’t soak up enough carbon to solve global warming, researchers say. However, it will provide an environmentally friendly, low-cost way to make nanoporous graphene for use in “supercapacitors”—devices that can store energy and release it rapidly. Such devices are used in everything from consumer electronics to heavy industry, and can be useful in hybrid electric vehicles and even in renewable energy storage systems.
Energy storage technologies have the potential to offset the intermittency problem of renewable energy sources by storing generated energy, making it accessible upon demand.
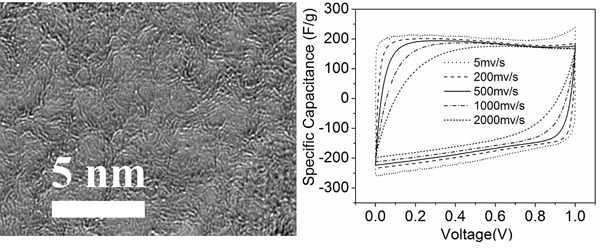
In the chemical reaction that was developed for the purpose of this study*, the end result is nanoporous graphene, a form of carbon that’s ordered in its atomic and crystalline structure. It has an enormous specific surface area of about 1,900 square meters per gram of material. Because of that, it also offers an electrical conductivity at least 10 times higher than the activated carbon now used to make commercial supercapacitors.
“There are other ways to fabricate nanoporous graphene, but this approach is faster, has little environmental impact and costs less,” said Xiulei (David) Ji, an OSU assistant professor of chemistry in the OSU College of Science, and lead author on the study. “The product exhibits high surface area, great conductivity and, most importantly, it has a fairly high density that is comparable to the commercial activated carbons.
“And, the carbon source is carbon dioxide, which is a sustainable resource, to say the least,” Ji added. “This methodology uses abundant carbon dioxide, while making energy storage products of significant value.”
Because the materials involved are inexpensive and the fabrication is simple, this approach has the potential to be scaled up for production at commercial levels, according to Ji.
The chemical reaction outlined in this study involved a mixture of magnesium and zinc metals—a combination discovered for the first time. These metals were heated to a high temperature in the presence of a flow of carbon dioxide, so as to produce a controlled “metallothermic” reaction. The reaction converted the elements into their metal oxides and nanoporous graphene, a pure form of carbon that’s remarkably strong. It can also efficiently conduct heat and electricity. The metal oxides can later even be recycled back into their metallic forms to make the industrial process more efficient.
By comparison, other methods to make nanoporous graphene often use corrosive and toxic chemicals, in systems that would be challenging to use at large commercial levels.
“Most commercial carbon supercapacitors now use activated carbon as electrodes, but their electrical conductivity is very low,” Ji said. “We want fast energy storage and release that will deliver more power, and for that purpose the more conductive nanoporous graphene will work much better. This solves a major problem in creating more powerful supercapacitors.”
A supercapacitor is a type of energy storage device, but it can be recharged much faster than a battery and has a great deal more power. They are mostly used in any type of device where rapid power storage and short, but powerful energy release is needed.
Aside from being used in consumer electronics, they have applications in heavy industry with the ability to power anything from a crane to the emergency slides on an aircraft. A supercapacitor can also capture energy that might otherwise be wasted, such as in braking operations. They stand to greatly improve the efficiency of hybrid electric automobiles.
Perhaps, most notably, their energy storage abilities may help “smooth out” the power flow of intermittent renewable sources, such as from wind power systems. Due to the variable nature of the wind, combining energy storage sources can allow for more reliable wind power integration (even when the winds are calm).
The use for supercapacitors is continually expanding. For instance, their Nanoporous carbon materials can adsorb gas pollutants, work as environmental filters, and be used in water treatment. Progress has really only been constrained by their cost so far.
* The findings were recently published in “Nano Energy” by scientists from the OSU College of Science, OSU College of Engineering, Argonne National Laboratory, the University of South Florida, and the National Energy Technology Laboratory in Albany, Oregon (the work was supported by OSU).
Oregon State University (OSU) is one of only two universities in the US that is designated a Land Grant, Sea Grant, Space Grant, and Sun Grant institution. OSU is also Oregon’s only university to hold the Carnegie Foundation’s top designation for research institutions and its prestigious Community Engagement classification.
Oregon State University (OSU)
http://oregonstate.edu
Author: David Stauth
Volume: January/February 2015











